-
OSANG HEALTHCARE Lab.
-
Biochemical Diagnostics
Division -
Immunodiagnosis Business
Division -
Molecular Diagnostics
Division
The OSANG HEALTHCARE Research Center consists of the Research Support Division, which oversees R&D management across all business units, the Biochemical Sensor Development Division, which focuses on cartridges, strips, and cassettes, and the Instrument Development Division, which handles devices, analyzers, and production systems.
-
Research Support Division
The Research Support Division operates digital systems for R&D management, maintaining a comprehensive database of resources such as equipment, instruments, reagents, and consumables. This enables rapid development cycles and ensures traceability in compliance with regulatory requirements. The division also manages national research projects, including personnel and budget.
-

Electronic Lab Notebook (BIOVIA)
Digital registration and management of DHF documents and project tracking
Electronic Lab Notebook (BIOVIA)
-

Resource Management System (CISPro)
Inventory management for reagents, instruments, and other R&D resources, integrated with the electronic lab notebook
Resource Management System (CISPro)
-

Government Project Management
Oversight of project manpower and budget allocation
Government Project Management
-
-
Biochemical Sensor Development Division
This division develops diagnostic platforms for biochemistry, immunoassay, molecular diagnostics, and continuous glucose monitoring, enabling a versatile product portfolio adaptable to market needs.
-
Diabetes Diagnostics
Development of immuno/chemical/enzyme-based reagents and cartridges for diabetes and related complications
Diabetes Diagnostics
-
Chronic Disease Diagnostics
Reagents and cartridges for detecting biomarkers of chronic diseases
Chronic Disease Diagnostics
-

Infectious Disease Diagnostics
Molecular and immuno-based reagents and cartridges/cassettes for infectious diseases
Infectious Disease Diagnostics
-
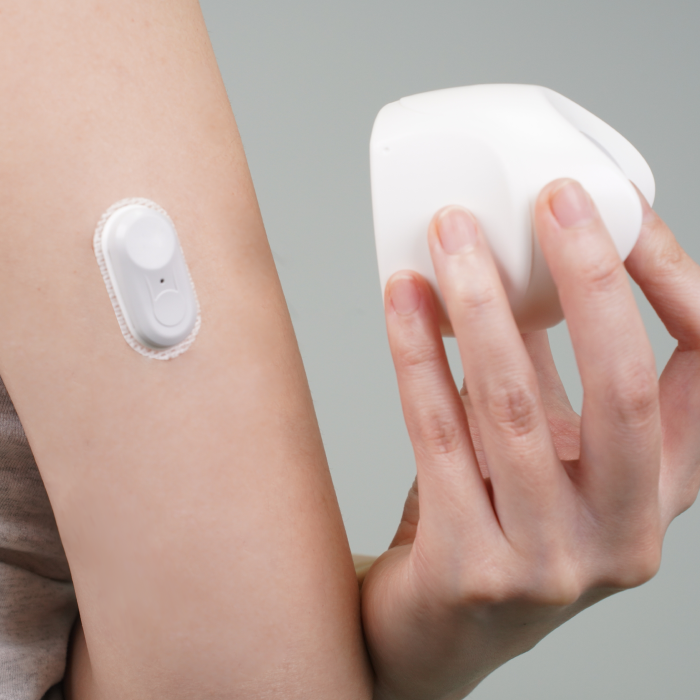
CGMS Development
R&D and production automation systems for continuous glucose monitoring
CGMS Development
-
-
Instrument Development Division
This division develops systems that operate with various test formats (cartridge, strip, cassette) across multiple detection principles. Comprising experts in mechanics, hardware, software, firmware, and server infrastructure, the team also designs production automation systems.
-
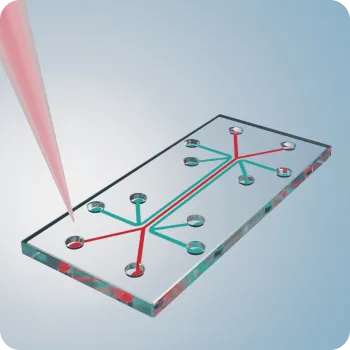
All-in-One Cartridge Design
Mechanical systems for liquid and dry reagent integration with optical measurement
All-in-One Cartridge Design
-

Sample-to-Answer Systems
Automation and algorithm development to minimize user intervention
Sample-to-Answer Systems
-
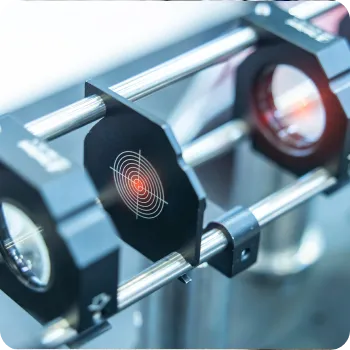
Multi-Detection Optical Systems
Platforms for detecting immuno/chemical/enzyme-based signals
Multi-Detection Optical Systems
-

Software/Firmware Development
Operation and data management software and firmware
Software/Firmware Development
-

Hospital Network Systems
LIS/HIS-compatible server and database integration for hospital use
Hospital Network Systems
-

Automation for Mass Production
Systems for automated manufacturing of cartridges, strips, and cassettes
Automation for Mass Production
-
The Biochemistry Division sets new standards in early detection and health management through blood-based diagnostic technology, providing accurate and reliable in-vitro diagnostic solutions.
-
Biochemical Diagnostics
By measuring key biomarkers such as glucose and glycated hemoglobin (HbA1c) in blood, it plays a critical role in the early detection and management of diabetes, insulin resistance, and related metabolic disorders.
-

Blood Testing System Development
Testing blood samples for glucose, β-ketones, cholesterol, HbA1c, and other biomarkers.
Blood Testing System Development
-

Monitoring Platform
Application and DM Server Integration Program
Monitoring Platform
-

Chronic Disease Management System
Development of Diagnostic Systems for Chronic Disease Prevention and Management
Chronic Disease Management System
-
The Immunodiagnostics Division oversees the full cycle of IVD medical devices based on immunoassay technologies—from planning and development to mass production.
-
Immunoassay
Immunoassays utilize antigen-antibody binding to detect minute amounts of biomarkers in blood samples.
-

Respiratory Diseases
Diagnostic products for conditions such as COVID-19 and influenza
Respiratory Diseases
-
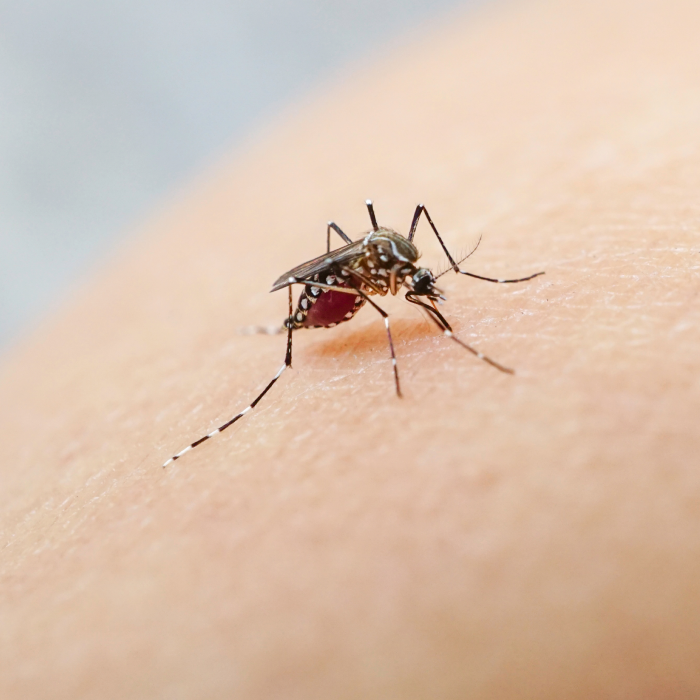
Mosquito-Borne Diseases
Products for vector-borne illnesses such as dengue and Zika virus
Mosquito-Borne Diseases
-

Other Applications
Diagonostics for hormonal markers and other indicators
Other Applications
-
Specializing in IVD solutions based on real-time PCR,
this division focuses on infectious disease detection and genotyping.
Its structured organization covers planning, R&D, production,
and quality control to ensure reliable diagnostics.
-
Molecular Diagnostics
Molecular diagnostics analyze nucleic acids extracted from human samples
to detect specific genes such as HPV or HLA, supporting disease diagnosis and prognosis.-
Infectious Disease Detection
Development of reagents for real-time amplification and detection of pathogens
Infectious Disease Detection
-

Genotyping
Reagents for identifying gene mutations or genetic variations
Genotyping
-