Molecular Diagnosis
Molecular diagnosis can detect human papilloma virus (HPV), and enable testing for histocompatibility (HLA) and infectious diseases, by examining nucleic acid (DNA and RNA) for genetic information (e.g., on a virus or human body).
-
GeneFinder™ Flu A&B RealRealAmp Kit
-
GeneFinder™ HLA-B*57:01 RealRealAmp Kit
-
GeneFinder™ HPV-HR RealRealAmp Kit
-
GeneFinder™ COVID-19 Fast RealRealAmp Kit
-

GeneFinder™ COVID-19/Flu A&B RealRealAmp Kit
-

GeneFinder™ COVID-19 Plus RealRealAmp Kit
-
GeneFinder™ HLA-ABCDRB1DQ RealAmp Kit
-

GeneFinder™ HLA-ABDR RealAmp Kit
-

GeneFinder™ HLA-B*27 RealAmp Kit
-
GeneFinder™ HLA-B*51 RealAmp Kit
-

GeneFinder™ HPV PCR Kit
-
GeneFinder™ HPV LBMA Genotype Kit
-

GeneFinder™ STDⅠ(CT/NG/UU) Multiplex Real-time PCR Kit
-

GeneFinder™ STDⅡ(MG/MH/TV) Multiplex Real-time PCR Kit
-

GeneFinder™ TB & NTM Multiplex Real-time PCR Kit
-

GeneFinder™ Malaria RealAmP Kit
-
GeneFinder™ DENV/CHKV RealAmp kit
-

GeneFinder™ DENV Typing RealAmp kit
GeneFinder™ Flu A&B RealAmp Kit
CE-IVD
GeneFinder Flu A&B RealAmp Kit is a qualitative kit and detect the RNA of Influenza A virus, Influenza B virus and Influenza A virus subtype (H1N1/09/H3N2) from nasopharyngeal swab (NPS) by using Real-time PCR system.
Features
-
About 120 minutes to simultaneously detect multiple targets in a single tube
-
Reverse Transcription reaction and Real-time Polymerase Chain Reaction
-
Easy-to-use (One-Tube) and interpretation
-
Reliable resuly by Internal/Positive/Negative Control
-
CE-IVD
-
MFDS MFDS License No. IVD-18-749 (Export Only)
Specimens
– Respiratory Specimen (Nasopharyngeal swab)
Analytes
– Influenza A virus
– Influenza B virus
– Influenza A subtype H1N1/09
– Influenza A subtype H3N2
Compatible instruments
– Applied Biosystems 7500/7500 Fast (Thermo Fisher Scientific)
– CFX96 (Bio-Rad)
– QuantStudio 5 (Thermo Fisher Scientific)
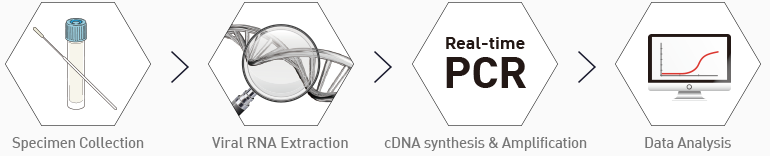
Working Process
Ordering information
| Product | Content | Catalog No. | Compatible instruments | Size |
|---|---|---|---|---|
| GeneFinder™ Flu A&B RealAmp Kit | Flu A&B Reaction Buffer Flu A&B Enzyme Mixture Flu A&B Probe Mixture Flu A&B Positive Control Flu A&B Negative Control |
IFMR-28.02B100 IFMR-28-I.02B100 |
CFX96 Applied Biosystems 7500/7500 Fast QuantStudio 5 |
100 Tests/Kit |
GeneFinder™ HLA-B*57:01 RealAmp Kit
CE-IVD
Abacavir is a nucleoside reverse transcriptase inhibitor with activity against the human immunodeficiency virus (HIV). The abacavir hypersensitivity reaction is a multiorgan clinical syndrome typically seen within the initial 6 weeks of abacavir treatment.
In approximately 5-9% Caucasian of treated patients, abacavir can induce an immune mediated hypersensitive response that correlates with the presence of the HLA-B*57:01 allele Consequently, current guidelines recommend screening for the presence of the HLA-B*57:01 allele in all HIV infected patients before abacavir initiation
Features
-
Within 90 minutes detection for B*57:01 and B*57 screening
-
Easy-to-use master mix type
-
Reliable result by internal / positive / negative control
-
Including complete assay kits
-
CE-IVD approved
Specimens
– Whole blood
Analytes
– HLA-B*57:01
– HLA-B*57
Compatible instruments
– Applied Biosystems 7500/7500 Fast (Thermo Fisher Scientific)
– CFX96 (Bio-Rad)
– QuantStudio 5 (Thermo Fisher Scientific)
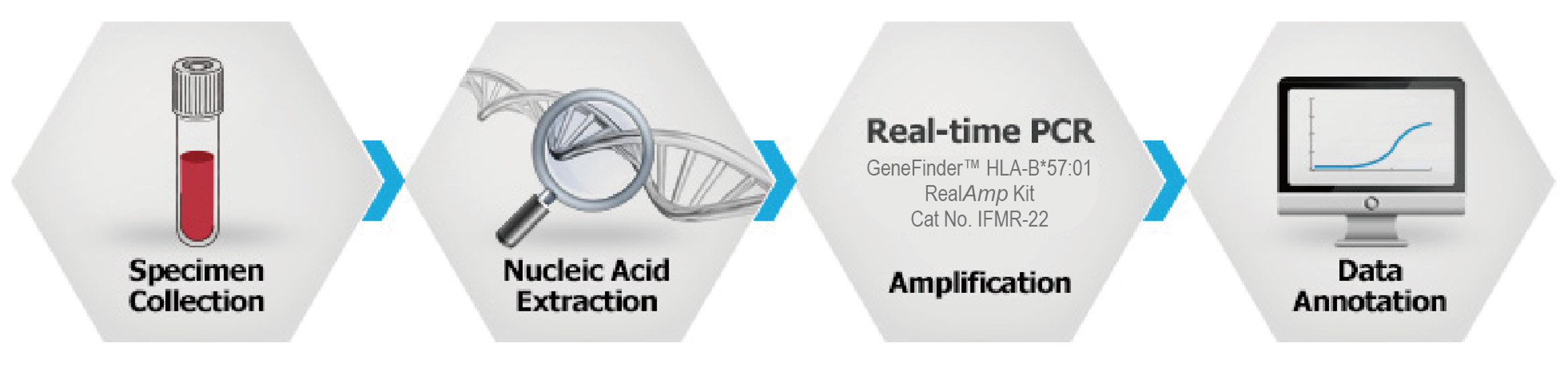
Working Process
Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ HLA-B*57:01 RealAmp Kit | B5701 Rxn B5701 DNA Pol. B5701 PC |
IFMR-22 | 50 Tests/Kit |

GeneFinder™ HPV-HR RealAmp Kit
CE-IVD
GeneFinder HPV-HR RealAmp Kit is real-time polymerase chain reaction (RT-PCR) test for the qualitative detection of 14 high-risk HPV type (#16, #18, #31, #33, #35, #39, #45, #51, #52, #56, #58, #59, #66 and #68) at high risk of developing cervical cancer from cervical specimens by using Real-time PCR system.
Features
-
About 120 minutes to simultaneously detect multiple targets in a single tube
-
Real-time Polymerase Chain Reaction
-
Easy-to-use (One-Tube) and interpretation
-
Reliable result by internal / positive / negative control
-
CE-IVD
-
MFDS License No. IVD-19-38 (Export Only)
Specimens
– Cervical Specimens
Analytes
– HPV Type 16
– HPV Type 18
– HPV Type 31, 33, 35, 39, 45, 51, 52,56, 58, 59, 66, 68
Compatible instruments
– Applied Biosystems 7500/7500 Fast (Thermo Fisher Scientific)
– CFX96 (Bio-Rad)
– InGenius (ELITech)
– Gentier96E (TIANLONG)
Working Process
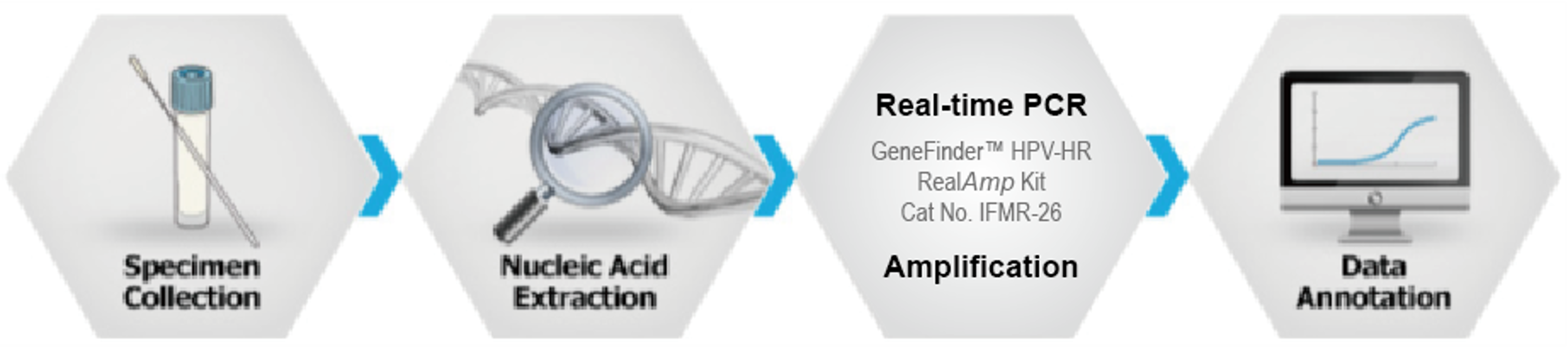
Ordering information
| Product | Content | Catalog No. | Compatible instruments | Size |
|---|---|---|---|---|
| GeneFinder™ HPV-HR RealAmp Kit | HPV-HR Reaction Mixture HPV-HR Probe Mixture HPV-HR Positive Control HPV-HR Negative Control |
IFMR-26.02B100 | Applied Biosystems 7500/7500 Fast – CFX96 – InGenius – Gentier 96E |
100 Tests/Kit |
GeneFinder™ COVID-19 Fast RealAmp Kit
CE-IVD
The Chinese authorities identified a new type of coronavirus (SARS-CoV-2, named as COVID-19), which was isolated on 7 January 2020. On January 11, 2020, Chinese health authorities preliminarily identified more than 40 human infections with a novel coronavirus in an outbreak of pneumonia under investigation in Wuhan City, Hubei Province, China.
GeneFinder™ COVID-19 Fast RealAmp Kit is the One-Step Reverse Transcription Real-Time PCR Kit designed to detect Novel Corona virus (COVID-19) qualitatively through Reverse Transcription reaction and Real-Time Polymerase Chain Reaction.
Features
-
About 50 minutes detection for COVID-19 in a single tube
-
Reverse Transcription reaction and Real-Time Polymerase Chain Reaction
-
Easy-to-use (One-Tube) and interpretation
-
Reliable resuly by Internal/Positive/Negative Control
-
CE-IVD
-
MFDS License No. IVD-20-788 (Export Only)
Specimens
– Respiratory Specimen (Nasopharyngeal & Oropharyngeal (Throat) swab, Sputum and etc.)
Analytes
– COVID-19 by amplifying the RdRp, E and N gene
Compatible instruments
– Applied Biosystems 7500 Fast (Thermo Fisher Scientific)
– CFX96 (Bio-Rad)
– QuantStudio 5 (Thermo Fisher Scientific)
Working Process

Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ COVID-19 Fast RealAmp Kit | COVID-19 Fast Reaction Buffer COVID-19 Fast Enzyme Mixture COVID-19 Fast Probe Mixture COVID-19 Fast Positive Control COVID-19 Fast Negative Control DEPC Water |
IFMR-49 | 100 Tests/Kit |

GeneFinder™ COVID-19 Fast RealAmp Kit
CE-IVD
The Chinese authorities identified a new type of coronavirus (SARS-CoV-2, named as COVID-19), which was isolated on 7 January 2020. On January 11, 2020, Chinese health authorities preliminarily identified more than 40 human infections with a novel coronavirus in an outbreak of pneumonia under investigation in Wuhan City, Hubei Province, China.
GeneFinder™ COVID-19 Fast RealAmp Kit is the One-Step Reverse Transcription Real-Time PCR Kit designed to detect Novel Corona virus (COVID-19) qualitatively through Reverse Transcription reaction and Real-Time Polymerase Chain Reaction.
Features
-
About 50 minutes detection for COVID-19 in a single tube
-
Reverse Transcription reaction and Real-Time Polymerase Chain Reaction
-
Easy-to-use (One-Tube) and interpretation
-
Reliable resuly by Internal/Positive/Negative Control
-
CE-IVD
-
MFDS License No. IVD-20-788 (Export Only)
Specimens
– Respiratory Specimen (Nasopharyngeal & Oropharyngeal (Throat) swab, Sputum and etc.)
Analytes
– COVID-19 by amplifying the RdRp, E and N gene
Compatible instruments
– Applied Biosystems 7500 Fast (Thermo Fisher Scientific)
– CFX96 (Bio-Rad)
– QuantStudio 5 (Thermo Fisher Scientific)
Working Process

Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ COVID-19 Fast RealAmp Kit | COVID-19 Fast Reaction Buffer COVID-19 Fast Enzyme Mixture COVID-19 Fast Probe Mixture COVID-19 Fast Positive Control COVID-19 Fast Negative Control DEPC Water |
IFMR-49 | 100 Tests/Kit |
GeneFinder™ COVID-19/Flu A&B RealAmp Kit
CE-IVD
GeneFinder™ COVID-19/Flu A&B RealAmp Kit is the One-Step Reverse Transcription Real-Time PCR Kit designed to detect both Novel Corona virus (SARS-CoV-2, COVID-19) and Influenza A&B virus qualitatively through Reverse Transcription reaction and Real-Time Polymerase Chain Reaction.
Features
-
About 120 minutes detection for both COVID-19 and Influenza A&B in a single tube
-
Reverse Transcription reaction and Real-Time Polymerase Chain Reaction
-
Easy-to-use(One-Tube) and interpretation
-
Reliable result by Internal/Positive/Negative Control
-
CE-IVD
-
MFDS License No. IVD-20-986 (Export Only)
Specimens
– Respiratory Specimen (Nasopharyngeal & Oropharyngeal (Throat) swab, Sputum and etc.)
Analytes
– COVID-19 and Influenza A&B
Compatible instruments
-Applied Biosystems 7500/7500 Fast (Thermo Fisher Scientific)
– CFX96(Bio-Rad)
– QuantStudio 5 (Thermo Fisher Scientific)
Working Process

Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ COVID-19/Flu A&B RealAmp Kit | COVID-19/Flu A&B Reaction Mixture COVID-19/Flu A&B Probe Mixture COVID-19/Flu A&B Positive Control COVID-19/Flu A&B Negative Control |
IFMR-57 | 100 Tests/Kit |

GeneFinder™ COVID-19 Plus RealAmp Kit
FDA EUA CE-IVD
On January 11, 2020, Chinese health authorities preliminarily identified more than 40 human infections with a novel coronavirus in an outbreak of pneumonia under investigation in Wuhan City, Hubei Province, China. The Chinese authorities identified a new type of coronavirus (novel coronavirus, named as COVID-19), which was isolated on 7 January 2020.
GeneFinder™ COVID-19 Plus RealAmp Kit is the One-Step Reverse Transcription Real-Time PCR Kit designed to detect Novel Corona virus (COVID-19) qualitatively through Reverse Transcription reaction and Real-Time Polymerase Chain Reaction.
Features
-
About 120 minutes detection for COIVD-19 in a single tube
-
Reverse Transcription reaction and Real-Time Polymerase Chain Reaction
-
Easy-to-use(One-Tube) and interpretation
-
Reliable result by Internal/Positive/Negative Control
-
FDA EUA
-
CE-IVD
-
License NO. 20-196
Specimens
– Respiratory Specimen
(Sputum, Nasopharyngeal swab,
Oropharyngeal swab)
Analytes
– COVID-19
Compatible instruments
– Applied Biosystems 7500/7500FAST(Thermo fisher)
– CFX96(Bio-Rad)
– For application of InGenius, please contact Elitech Group S.p.A (www.elitechgroup.com) directly
Working Process

Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ COVID-19 Plus RealAmp Kit | COVID-19 Plus Reaction Mixture COVID-19 Plus Probe Mixture COVID-19 Plus Positive Control COVID-19 Plus Negative Control |
IFMR-45 | 100 Tests/Kit |

GeneFinder™ HLA-ABCDRB1DQ RealAmp Kit
CE-IVD
HLA(human leukocyte antigen) is major gentietic marker for transplantation and specific disease. In most laboratories, conventional typing method was performed by cell cytotoxicity tests or fluorescence serology with specific antibodies, and now DNA typing method using PCR is widely being used due to its specificity. However, PCR typing method require labor and time for result analysis, therefore the new accurate typing method is needed for and labor saving.
HLA-ABCDRB1DQ RealAmp Kit provides fast, accurate and method for HLA typing based on real-time PCR platform. Primer sets for reaction are pre-aliquoted in a 96-well test plate for fast process and high throughput system. Provided auto analysis program(software) makes easy and accurate interpretation of result
Features
-
Within 90 minutes detection for HLA genotyping
-
SSP(Sequence Specific Primer) type based on Real-Time PCR technology
-
Convenient analysis of result via dedicated S/W
-
Easy-to-use and great interpretation
-
Pre-aliquoted primer sets in a 96-well plate for time saving
-
High throughput system
-
Including complete assay kits
Specimens
– Whole blood
Analysis
– Human Major histocompatibility complex (MHC) Class I and II
Compatible instruments
– Applied Biosystems 7500 / Fast (Life Technologies)

Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ HLA-ABCDRB1DQ RealAmp Kit | HLA-ABCDRB1DQ Rxn
HLA-ABCDRB1DQ Pol. HLA-ABCDRB1DQ 96 well plate |
IFMR-20 | 6 tests |
| Advertisement Review Confirmation : Review Number 2016-I10-32-1961 |
| For more information, please contact us |

GeneFinder™ HLA-ABDR RealAmp Kit
CE-IVD
HLA(human leukocyte antigen) is major gentietic marker for transplantation and specific disease. In most laboratories, conventional typing method was performed by cell cytotoxicity tests or fluorescence serology with specific antibodies, and now DNA typing method using PCR is widely being used due to its specificity. However, PCR typing method require labor and time for result analysis, therefore the new accurate typing method is needed for and labor saving.
HLA-ABBDR RealAmp Kit provides fast, accurate and method for HLA typing based on real-time PCR platform. Primer sets for reaction are pre-aliquoted in a 96-well test plate for fast process and high throughput system. Provided auto analysis program(software) makes easy and accurate interpretation of result
Features
-
Within 90 minutes detection for HLA genotyping
-
SSP(Sequence Specific Primer) type based on Real-Time PCR technology
-
Convenient analysis of result via dedicated S/W
-
Easy-to-use and great interpretation
-
Pre-aliquoted primer sets in a 96-well plate for time saving
-
High throughput system
-
Including complete assay kits
-
CE-IVD / Ministry Food and Drug Safety (Korea) approved
Specimens
– Whole blood
Analysis
– Human Major histocompatibility complex (MHC) Class I and II
Compatible instruments
– Applied Biosystems 7500 (Life Technologies)
Working Process
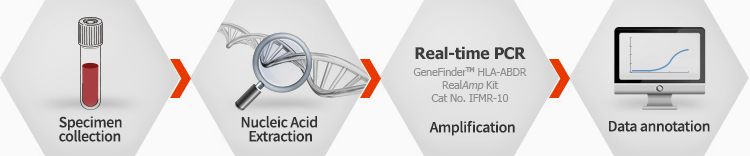
Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ HLA-ABDR RealAmp Kit | HLA-ABDR Rxn
HLA-ABDR Pol. HLA-ABDR 96 well plate |
IFMR-10 | 20 tests |
| Advertisement Review Confirmation : Review Number 2016-I10-32-1961 |
| For more information, please contact us |
GeneFinder™ HLA-B*27 RealAmp Kit
CE-IVD
The HLA-B*27, one of the HLA-B genotypes, is closely related to Ankylosing spondylitis (AS) which cause inflammation of the spondylathritis and pericoxitis such as inflammatory rheumatic disease. The 95% of Ankylosing spondylitis patients have the HLA-B*27 genes and normal person also has that 5 ~ 6%
HLA-B*27 RealAmp Kit is a qualitative using Real-time PCR technology, and highly reliable kit using the internal control.
Features
-
Within 90 minutes detection for B*27 screening
-
Easy-to-use master mix type
-
Reliable result by Internal/Positive/Negative Control
-
Including complete assay kits
-
CE-IVD / Ministry Food and Drug Safety (Korea) approved
Specimens
– Whole blood
Analytes
– HLA-B*27
Compatible instruments
– Applied Biosystems 7500 (Life Technologies)
– CFX 96TM (BIO-RAD)
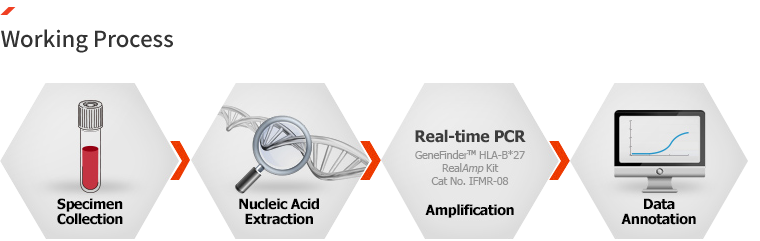
Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ HLA-B*27 RealAmp Kit | B27 2X Rxn B27 DNA Pol. B27 PC |
IFMR-08 | 100 tests |
| Advertisement Review Confirmation : Review Number 2015-I10-29-1653 |
| For more information, please contact us |
GeneFinder™ HLA-B*51 RealAmp Kit
CE-IVD
Bechet’s disease (BD) is a chronic inflammatory disorder, involving several organs. Althoungh the exact pathogenesis for BD is not completely understood, it has been suggested that the disease is triggered in genetically susceptible individuals by environmental factors, such as microbial agents. According to the reports, HLA-B*51 alleles which are responsible for immune function of human leukocyte antigen have been reported that patients of Behcet’s disease were observed highly concentration than normal.
HLA-B*51 RealAmp Kit is a qualitative in vitro diagnostic Kit using real-time PCR system, and simple convenient test detecting HLA-B*51 genes.
Features
-
Within 90 minutes detection for B*27 screening
-
Easy-to-use master mix type
-
Reliable result by Internal/Positive/Negative Control
-
Easy-to-use and great interpretation
-
Including complete assay kits
-
CE-IVD / Ministry Food and Drug Safety (Korea) approved
Specimens
– Whole blood
Analytes
– HLA-B*51
Compatible instruments
– Applied Biosystems 7500 (Life Technologies)
– CFX 96TM (BIO-RAD)

Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ HLA-B*51 RealAmp Kit | B51 2X Rxn B51 DNA Pol. B51 PC |
IFMR-09 | 100 tests |
| Advertisement Review Confirmation : Review Number 2015-I10-29-1650 |
| For more information, please contact us |
GeneFinder™ HPV PCR Kit
CE-IVD
Human Papilloma Virus (HPV) can cause human genital infections, of which 40 types are known. Specifically high-risk HPV type can leads cervical cancer, and also other type can cause various disease such as larygeal papillomatosis or warts.
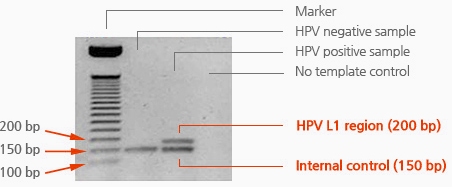
HPV PCR Kit is designed to amplify HPV L1 region based on one-tube nested. It is set to asymmetric PCR condition for hybridization without denaturation of PCR products as well.
Features
-
Available to detect HPV L1 region
-
One-tube nested PCR
Specimens
– Cervical swab
– Vaginal discharge
Analytes
– HPV L1 region
Compatible instruments
– PCR machine device
Performance data
Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ HPV PCR Kit | HPV Reaction Mixture HPV Primer Mixture I/II HPV Positive Control I/II HPV Negative Control |
IFMR-01 | 100 tests |
| Advertisement Review Confirmation : Review Number 2015-I10-29-1651 |
| For more information, please contact us |
GeneFinder™ HPV LBMA Genotype Kit
CE-IVD
Human Papilloma Virus (HPV) can cause human genital infections, of which 40 types are known. Specifically high-risk HPV type can lead cervical cancer, and also other type can cause various disease such as larygeal papillomatosis or warts.
HPV LBMA is designed to 32 types of HPVs(high-risk and low-risk HPV) including high-risk and low-risk fast and accurately bases on liquid bead microarray platform using PCR cycler and Luminex® analyzer. Target DNAs are amplified by One Tube Nested (OTN) and asymmetric PCR technology (refer to HPV PCR Kit (Cat no. IFMR-01), and it bind with dual fluorescence of different ratio within microbead. The targets are detected through the laser and analyzed using the proprietary program automatically and accurately.
Features
-
Multplex PC & Liquid Bead MicroArray (LBMA) platform
-
Detection of the genotype of 19 types of high risks and 13 types of low risks at once with Luminex instrument
-
Easy-to-use system: Unique analyzing program to analyze the HPV test result
-
High accuracy & High throughput system
-
CE-IVD / Ministry Food and Drug Safety (Korea) approved
Analytes
| Type of risk | Genotype |
|---|---|
| High | 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 69, 70, 73 |
| Low | 6, 11, 32, 34, 40, 42, 43, 44, 54, 55, 62, 81, 83 |
Specimens
– Cervical swab
– Vaginal discharge
Compatible instruments
– Luminex®200™ System (Luminex)
– Partnership with Luminex(since Dec 2013)
Working Process

Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ HPV LBMA Genotype Kit | GF Buffer A GF Buffer B GF Bead |
IFMR-02 | 100 tests |
| Advertisement Review Confirmation : Review Number 2015-I10-29-1652 |
| For more information, please contact us |

GeneFinder™ STD Ⅰ(CT/NG/UU) Multiplex Real-time PCR Kit
CE-IVD
Sexually transmitted diseases (STD), also referred to as Sexually transmitted infections(STIs) and venereal diseases (VD), are illnesses that have a significant probability of transmission between humans by means of human sexual behavior. Some such infections can also be transmitted non-sexually, such as from mother to infant during pregnancy or childbirth, or through blood transfusions or shared needles.
STD I(CT/NG/UU) Multiplex Real-time PCR Kit is designed for detection of DNA of Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), Ureaplasma urealyticum (UU) in a single reaction using DNA samples.
Features
-
Multiplex Real-Time PCR type
-
90 minutes detection of various targets simultaneously in a single tube
-
HotStart PCR system: High specificity and sensitivity
-
Uracil-N-glycosylase(UNG) system: No carryover contamination
-
Reliable result by internal/positive/negative control
-
Easy to use master mix type
-
Including complete assay kits
-
CE-IVD / Ministry Food and Drug Safety (Korea) approved
Specimens
– Urogenital swab
– Urine
Analytes
– Chlamydia trachomatis (CT)
– Neisseria gonorrhoeae (NG)
– Ureaplasma urealyticum(UU)
Compatible instruments
– Applied Biosystems 7500 (Life Technologies)
Working Process
Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ STD I (CT/NG/UU) Multiplex Real-time PCR Kit | STD I Reaction Mixture STD I Probe Mixture STD I Positive Control STD I Negative Control |
IFMR-03 | 100 tests |
| Advertisement Review Confirmation : Review Number 2014-I10-29-2031 |
| For more information, please contact us |
GeneFinder™ STD Ⅱ (MG/MH/TV) Multiplex Real-time PCR Kit
CE-IVD
Sexually transmitted diseases (STD), also referred to as Sexually transmitted infections(STIs) are illnesses that have a significant probability of transmission between humans by means of human sexual behavior. Some such infections can also be transmitted non-sexually, such as from mother to infant during pregnancy or childbirth, or through blood transfusions or shared needles.
STD II(MG/MH/TV) Multiplex Real-time PCR Kit is designed for detection of DNA of Mycoplasma genitalium (MG), Mycoplasma hominis (MH), and Trichmonas vaginalis (TV) in a single reaction using DNA samples.
Features
-
Multiplex Real-Time PCR type
-
90 minutes detection of various targets simultaneously in a single tube
-
HotStart PCR system: High specificity and sensitivity
-
Uracil-N-glycosylase(UNG) system: No carryover contamination
-
Reliable result by internal/positive/negative control
-
Easy to use master mix type
-
Including complete assay kits
-
CE-IVD / Ministry Food and Drug Safety (Korea) approved
Specimens
– Urogenital swab
– Urine
Analytes
– Mycoplasma Genitalium (MG)
– Mycoplasma Hominis (MH)
– Trichomonas vaginalis (TV)
Compatible instruments
– Applied Biosystems 7500 (Life Technologies)
Working Process
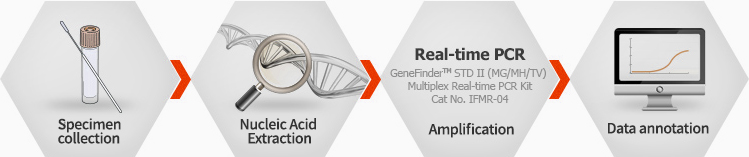
Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ STD II (MG/MH/TV) Multiplex Real-time PCR Kit | STD II Reaction Mixture STD II Probe Mixture STD II Positive Control STD II Negative Control |
IFMR-04 | 100 tests |
| Advertisement Review Confirmation : Review Number 2014-I10-29-2032 |
| For more information, please contact us |
GeneFinder™ TB & NTM Multiplex Real-time PCR Kit
CE-IVD
Tuberculosis(TB) is a disease caused by an infection with the bacteria Mycobacterium tuberculosis complex, It is highly contagious through the air, and can remain in an inactive state for years whithout causing symptoms. On the other hands, the non-tuberculous mycobacteria (NTM) refers to all the species in the family of mycobacteria that not cause human disease. Since Mycobacterium is difficult to culture and needs to be distinguished for diagnosis, it is highly important to be detected and differentiated based on PCR method.
TB & NTM Multiplex Real-time PCR Kit is designed to identify TB and NTM simultaneously within 90 minutes. Therefore, appropriate treatment can be made for the patient in time.
Features
-
Multiplex Real-Time PCR type
-
90 minutes detection of various targets simultaneously in a single tube
-
HotStart PCR system: High specificity and sensitivity
-
Uracil-N-glycosylase(UNG) system: No carryover contamination
-
Reliable result by internal/positive/negative control
-
Easy to use master mix type
-
Including complete assay kits
-
CE-IVD / Ministry Food and Drug Safety (Korea) approved
Specimens
– Sputum
– Bronchial washing
– Bronchoalveolar Lavage (BAL)
Analytes
– Mycobacterium tuberculosis (TB)
– Non-tuberculous mycobacteria (NTM)
Compatible instruments
– Applied Biosystems 7500 / Fast (Life Technologies)
– CFX 96™ (Bio-Rad)
Working Process
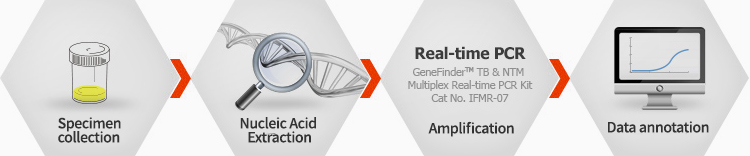
Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ TB & NTM Multiplex Real-time PCR Kit | TB & NTM Reaction Mixture TB & NTM Probe Mixture TB & NTM Positive Control TB & NTM Negative Control |
IFMR-07 | 100 tests |
| Advertisement Review Confirmation : Review Number 2014-I10-29-2035 |
| For more information, please contact us |

GeneFinder™ Malaria RealAmP Kit
CE-IVD
Malaria is a mosquito-borne infectious disease of human and other animals caused parasitic protozoans being to the genes Plasmodium.
Malaria parasites are transmitted to humans by the bite of infected female mosquitos of more than 30 anopheline spp. According to the latest estimates, there were about 198 million cause of malaria in 2013 (with an uncertainty rage of 124 million to 283 million) and an estimated 584,000 deaths (with and uncertainty rage 367,000 to 755,000). Malaria is caused by five species of parasites of the genus plasmodium that affect humans (P.falciparum, P.vivax, P.ovale, P.malariae and P.knowlesi) Mararia due to P.falciparum is the most deadly form and P.vivax is less dangerous but more widespread.
The other three species are found much less frequently. In the laboratory, malaria is diagnosed using different techniques.(e,g. Thin/thick blood smear, rapid diagnostic tests etc.). These methods showed relativity and low specificity than real time PCR method. Therefore, if you want to select accurate diagnostic product of malaria, Malaria RealAmp Kit is best choice.
Features
-
Screening of 5 Plasmodium spp.
– Detection of all five currently known Human pathogen Plasmodium spp.
– P.falciparum , P.vivax, P.ovale, P.malariaw and P.knowlesi -
Simultaneous identification of P.vivax and P.falciparum
-
Easy-to-use system
– No need to prepare materials (reagents/buffers)
– User convenience -
Rapid data analysis (1.5 hours)
-
Hot start PCR technology
– High specificity & sensitivity
-
Prevention of carryover contamination
– Uracil-N-glycosylase (UNG) system
Specimens
– Whole blood
Analytes
– Plasmodium falciparum
– Plasmodium vivax
– Plasmodium ovale
– Plasmodium malariae
– Plasmodium knowlesi
Compatible instruments
– Applied Biosystems 7500 (Life Technologies)
Working Process
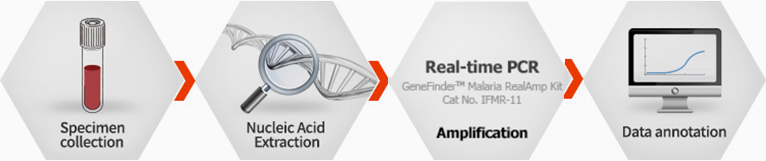
Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ Malaria RealAmp kit | Malaria Reaction Mixture Malaria Probe Mixture Malaria Positive Control Malaria Negative Control |
IFMR-11 | 100 tests |
GeneFinder™ DENV/CHKV RealAmp Kit
CE-IVD
Dengue fever is the most rapidly spreading mosquito-borne viral disease in the world. Dengue fever is acute fever disease caused by Dengue virus(DENV) in Flavivirus, genusand 4 serotypes of Dengue virus(DENV-1, DENV-2, DENV-3 and DENV-4) are known to cause human infection
Chikungunya fever is an emerging, mosquito-borne disease caused by an alphavirus, chikungunya virus(CHKV). The disease is transmitted predominantly by Aedes aegpti and Aedes albopictus mosquitoes, the same species involved in the transmission of dengue fever.
Although their routes of infection differ from one another the symptoms for both fevers are very similar that conventional diagnostics are inappropriate to distinguish them distinctively. Our GeneFinder ™ DENV/CHKV RealAmp kit is designed to identify infection from either DENV or CHKV distinctively and accurately based on One tube RT(Reverse Transcription)-PCR technique.
Features
-
DENV/CHKV RealAmp kit
– Detection of DENV/CHKV specific RNA
– Simultaneous differentiation of DENV and CHKV -
DENV/CHKV RealAmp kit / DENV Typing RealAmp kit
– Including IC (Internal Control) for confirmation of PCR Amplification / RNA Extraction process
– Ready-to-Use system
– One-tube RT (Reverse Transcription)-PCR technique
– Rapid data analysis (about 2hrs)
Specimens
– Whole Blood (w/EDTA)
– Plasma
– Serum
– CSF(Cerebrospinal fluid)
Analytes
– Human Major histocompatibility complex (MHC) Class I
Compatible instruments
– Applied Biosystems 7500 (Life Technologies)
– CFX 96™ (Bio-Rad)
Working Process

Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ DENV/CHKV RealAmp kit | DENVCHKV Reaction Buffer
DENVCHKV Enzyme Mixture DENVCHKV Probe Mixture DENVCHKV Positive Control DENVCHKV Negative Control |
IFMR-13 | 100 tests |

GeneFinder™ DENV Typing RealAmp Kit
CE-IVD
Dengue fever is the most rapidly spreading mosquito-borne viral disease in the world. Dengue fever is acute fever disease caused by Dengue virus(DENV) in Flavivirus, genusand 4 serotypes of Dengue virus(DENV-1, DENV-2, DENV-3 and DENV-4) are known to cause human infection
Chikungunya fever is an emerging, mosquito-borne disease caused by an alphavirus, chikungunya virus(CHKV). The disease is transmitted predominantly by Aedes aegpti and Aedes albopictus mosquitoes, the same species involved in the transmission of dengue fever.
GeneFinder™ DENV Typing RealAmp kit is a qualitative amplification assay and allows to detect and differentiate 4 serotypes of the Dengue virus (DENV-1, DENV-2, DENV-3 and DENV-4) extracted from human blood, plasma, serum and cerebrospinal fluid (CSF) by using real-time PCR based on One tube RT-PCR.
Features
-
DENV Typing RealAmp kit
– Identification of 4 currently known pathogenic DENV in human
-
DENV/CHKV RealAmp kit / DENV Typing RealAmp kit
– Including IC (Internal Control) for confirmation of PCR Amplification / RNA Extraction process
– Ready-to-Use system
– One-tube RT (Reverse Transcription)-PCR technique
– Rapid data analysis (about 2hrs)
Specimens
– Whole Blood (w/EDTA)
– Plasma
– Serum
– CSF(Cerebrospinal fluid)
Analytes
– Human Major histocompatibility complex (MHC) Class I
Compatible instruments
– CFX 96™ (Bio-Rad)
Working Process

Ordering information
| Product | Content | Catalog No. | Size |
|---|---|---|---|
| GeneFinder™ DENV Typing RealAmp kit | DENVCHKV Reaction Buffer
DENVCHKV Enzyme Mixture DENVCHKV Probe Mixture DENVCHKV Positive Control DENVCHKV Negative Control |
IFMR-13 | 100 tests |


